
Background
Multiple medications have been discovered to target cell surface receptors. These are programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1). These receptors are specifically selected because they are important targets for cancer therapy. There are some similarities between these receptors, but there are differences to note. For example, the PD-1 receptor is expressed on T cells, B cells, monocytes, dendritic cells, natural killer T cells, and regulatory T cells. On the other hand, PD-L1 is expressed on T cells, B cells, dendritic cells, macrophages, bone marrow-derived mast cells, and a few non-immune cells.
T-cell exhaustion is commonly characterized by the presence of PD-1. PD-1 expression is found in cancers such as tumor infiltrating lymphocytes. PD-L1 is commonly overexpressed in many different types of tumors, such as tumor-associated macrophages.
To understand how PD-1 and PD-L1 work in the human body, it is best to look at each medication’s mechanism of action. The similarities between PD-1 and (PD-L1) medications are that they are monoclonal antibodies, (MAB) and are all available in an injection dosage form. The doses utilized are specific to the indication that the medication is being used for. Here, we will go into specifics to provide an overview of these medications that target cancer cells.
Medications
Nivolumab is a human IgG4 MAB and was approved by the FDA in 2014. This medication binds to the PD-1 and stops the PD-L1 and programmed death ligand-2 (PD-L2) from interacting with each other, which ultimately allows the PD-1 pathway inhibition to occur. Nivolumab is commonly indicated for:
- Melanoma
- Non-small cell lung cancer (NSCLC)
- Malignant pleural mesothelioma
- Renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), and urothelial carcinoma
- Classical Hodgkin lymphoma (cHL)
- Squamous cell carcinoma of the head and neck (SCCHN)
- Esophageal, gastric, colorectal, and gastroesophageal junction cancer
- Esophageal adenocarcinoma
Nivolumab’s dose strengths are 40 mg/4 mL, 100 mg/10 mL, 120 mg/12 mL, and 240 mg/24 mL solution in a single-dose vial. The common dosages of this medication are 240 mg every two weeks and 480 mg every four weeks. It is important to be aware of the immune-mediated adverse reactions, infusion-related reactions, complications of allogeneic HSCT, and embryo-fetal toxicity associated with this medication. Multiple adverse reactions can occur such as fatigue, rash, musculoskeletal pain, nausea, vomiting, etc. Also, if it is being used with another agent, there are other adverse reactions to consider compared to when it’s being used as a single agent.
Pembrolizumab (approved by the FDA in 2014) is a human IgG4 kappa that targets the PD-1 receptor which is why it has a similar mechanism of action to nivolumab, as well as a similar adverse reaction profile. Pembrolizumab and nivolumab differ in their indications. Some pembrolizumab indications are:
- Melanoma
- NSCLC and (cHL)
- HCC, RCC, Merkel cell, cutaneous squamous cell, urothelial, and endometrial carcinoma
- Esophageal and gastric cancer
- Primary mediastinal large B-cell lymphoma (PMBCL)
- Head and neck squamous cell cancer (HNSCC)
- Microsatellite instability-high or mismatch repair deficient and colorectal cancer
- Cervical, tumor mutational burden-high (TMB-H), and triple-negative breast cancer
Typical doses in practice are 200 mg every 3 weeks or 400 mg every 6 weeks. The strengths of this medication offered are also 100 mg/4 mL (25 mg/mL) solution in a single-dose vial. The adverse effects profile is similar to nivolumab.
Atezolizumab was granted FDA approval in 2014 and is phage-derived human IgG1 MAB that blocks PD–L1. This medication works by blocking the interaction between PD-1 and B7.1, but it doesn’t induce antibody-dependent cytotoxicity. Some of its indications are:
- Urothelial carcinoma
- NSCLC, SCLC, and HCC
- Melanoma
Some of the dosage forms available are 840 mg/14 mL (60 mg/mL) and 1200 mg/20 mL (60 mg/mL) solution in a single-dose vial. Routinely, doses of 840 mg every 2 weeks, 1200 mg every 3 weeks, or 1680 mg every 4 weeks are utilized in practice. It is important to be aware of the immune-mediated adverse reactions, infusion-related reactions, complications of allogeneic HSCT, and embryo-fetal toxicity which is similar to PD-1 precautions. The common adverse effects seen are fatigue, decreased appetite, nausea, and cough.
Avelumab is IgG1 human MAB anti-PD-L1. This medication was FDA approved in 2016, and is indicated for:
- Merkel cell carcinoma (MCC)
- Urothelial carcinoma (UC)
- RCC
This medication does warrant premedication and is used as needed thereafter. The common doses seen utilized are 800 mg every 2 weeks. The adverse effect profile is dependent on the indication that it is used for, but it is similar to what patients on atezolizumab experience. The common dosage form seen is 200 mg/10 mL (20 mg/mL) solution in single-dose vial.
Durvalumab is a human MAB that targets PD-L1 and was given FDA approval in 2017. Some of this medication’s indications are:
- Unresectable, Stage III NSCLC
- Extensive-stage small cell lung cancer (ES-SCLC)
- Locally advanced or metastatic biliary tract cancer (BTC)
Some of the injection dosage forms available are in a 500 mg/10 mL (50 mg/mL) and 120 mg/2.4 mL (50 mg/mL) solution in a single-dose vial. Commonly, patients receive either a weight-based dose that is dependent on their current weight, or a fixed dose of 1,500 mg every four weeks. The common adverse effects that patients experience are cough, fatigue, nausea, pneumonitis, and upper respiratory tract infections.
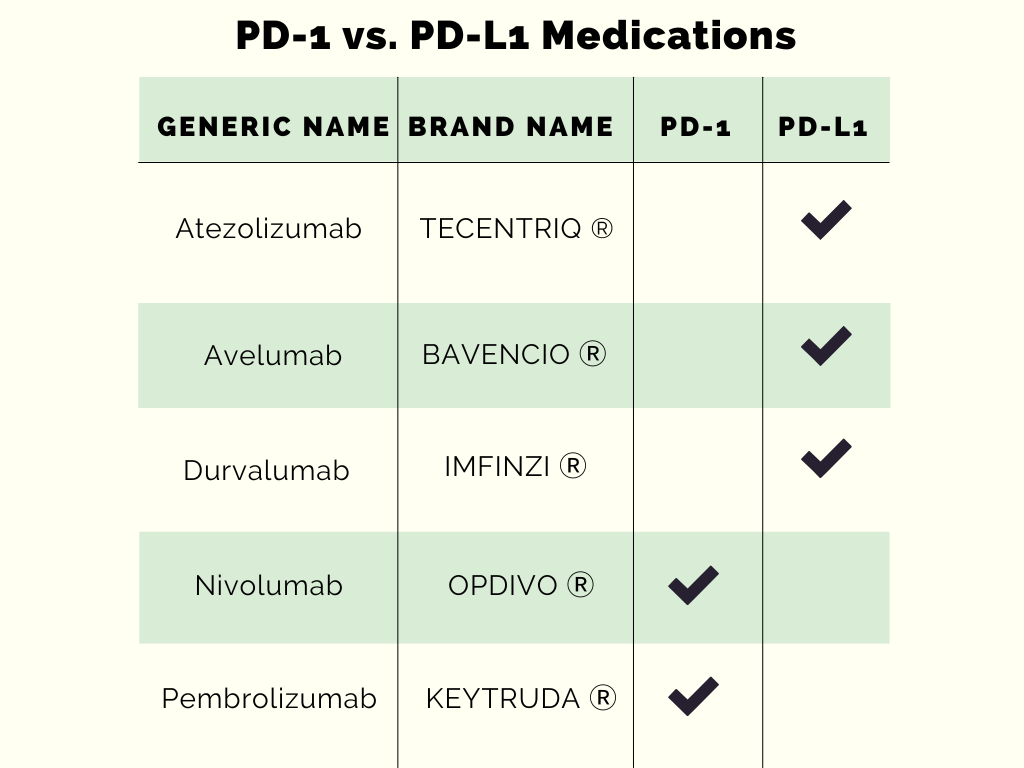
Overall, when comparing PD-1 and PD-L1 medications, examining their different indications, dosage strengths, and side effect profiles can be useful in determining the right therapy for the right patient. The over-expression of PD-1 and PD-L1 in cancer cells is the reason why these receptors have been targeted in studies to identify new medication options for different cancer types. It is important to continue to stay up to date with the latest developments in literature because new and old medications are continuously being studied to find new indications and breakthrough therapies.
-Dagmara Zajac
RxPharmacist Team
References:
- Tecentriq (atezolizumab) [prescribing information]. South San Francisco, CA: Genentech Inc; January 2022.
- Bavencio (avelumab) [prescribing information]. Rockland, MA: EMD Serono Inc; July 2022.
- Imfinzi (durvalumab) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; September 2022.
- Opdivo (nivolumab) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company; March 2022.
- Keytruda (pembrolizumab) [prescribing information]. Whitehouse Station, NJ: Merck & Co Inc; March 2022.
- Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15(5):1111-1122. doi:10.1080/21645515.2019.1571892
