
Direct oral anticoagulants (DOACs) are used to prevent thrombosis and are used for several different indications. This class of medication has stood out for several reasons, but the main reason was that this class of medication does not require regular laboratory monitoring compared to other anticoagulation drug classes. In many ways, anticoagulation guidelines and practices have changed with the addition of this therapeutic drug class. In 2010, the first DOAC dabigatran was approved by the Food and Drug Administration (FDA); since then, many more have been approved.
Dabigatran is categorized as the direct thrombin inhibitor DOAC compared to rivaroxaban and apixaban, which are categorized as the oral direct factor Xa inhibitor DOACs. By 2013, there were more prescriptions for DOACs compared to warfarin (vitamin K antagonist), which in previous years, was the commonly used form of anticoagulation by patients. The common FDA-approved indications for DOACs are the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), stroke prevention in nonvalvular atrial fibrillation (NVAF), prevention of recurrent DVT and PE, and many other indications. In addition, there are multiple off-label indications seen with this drug class as well.
There are considerations that need to be addressed when prescribing this class of medications. In terms of pharmacokinetics, there are comorbidities that may affect the efficacy of DOACs. For example, renal impairment, hepatic impairment, and body weight are all things to consider in patients initiating DOAC therapy. As mentioned previously, this drug class monitoring is different compared to previous methods of anticoagulation monitoring. Currently, there is no approved FDA-specific DOAC monitoring method; however, there are monitoring parameters that need to be performed in patients taking DOACs.
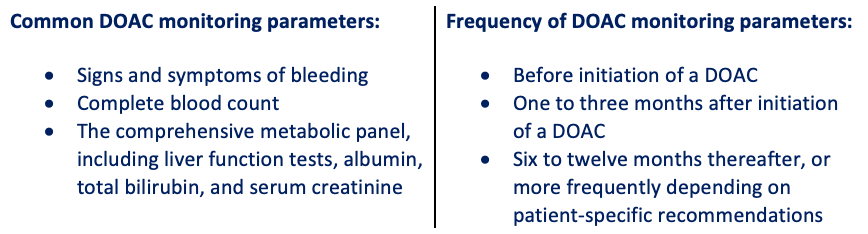
Patient education is critical when patients are beginning any anticoagulation medication. Anticoagulation medications are one of the therapeutic areas where a reversal agent for a class of medication is very beneficial due to the associated risk factors. When the first DOAC was approved, there was no reversal agent approved on the market. In 2015, FDA approved the first reversal agent for the first approved DOAC dabigatran. After that, in 2018, the next reversal agent was approved for apixaban and rivaroxaban.

For newer classes of medications, it is beneficial to be able to look at statistics to understand how receptive the healthcare systems and doctors are to adapting to new methods of treatment. The use of warfarin for atrial fibrillation between 2011 and 2020 decreased from 52.4% to 17.7%. In contrast, DOAC use for atrial fibrillation between 2011 to 2020 increased from 4.7% to 47.9%. It is apparent that these trends in oral anticoagulant use among patients with atrial fibrillation in community practice show an increased use of DOACs over warfarin.
In terms of anticoagulation methods for patients, DOACs have changed the standards of practice. Since the first DOAC was approved, there have been many advancements seen within this class of medications. This overview follows the current anticoagulant standards for patients. Perhaps in the near future, this drug class will continually expand and newer therapies will be introduced and implemented.
Dagmara Zajac
RxPharmacist Team
References:
- Chen A, Stecker E, A Warden B. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J Am Heart Assoc. 2020;9(13):e017559. doi:10.1161/JAHA.120.017559
- Navar AM, Kolkailah AA, Overton R, et al. Trends in Oral Anticoagulant Use Among 436 864 Patients With Atrial Fibrillation in Community Practice, 2011 to 2020. J Am Heart Assoc. 2022;11(22):e026723. doi:10.1161/JAHA.122.026723
- SavaysaÔ [package insert]. Basking Ridge, NJ: Daiichi Sankyo, Inc., 2021.
- Direct oral anticoagulants (doacs). Blood Clots. https://www.stoptheclot.org/about-clots/blood-clot-treatment/direct-oral-anticoagulants/. Published October 4, 2018. Accessed December 20, 2022.
