
Heart failure is a complex cardiac disease state that affects more than 6 million people in the United States. In patients with HF, the ability of the heart to eject blood into systemic circulation is decreased due to structural or functional changes that weaken the heart muscle. The approach to treating heart failure includes lifestyle modifications and a combination of medications. New pharmacotherapies continue to be developed and studied for the treatment of heart failure. The PARADIGM-HF trial was a large clinical trial that introduced a new class of medication and ultimately led to a change in the 2022 update of the heart failure treatment guidelines.
What was the PARADIGM-HF trial?
The PARADIGM-HF trial was a randomized, double-blind trial comparing an experimental drug containing sacubitril and valsartan to enalapril, a first-line treatment option, in patients who had heart failure with reduced ejection fraction (HFrEF).
The combination drug studied in this trial is now known by its brand name, Entresto. Entresto is the first drug in a new class called angiotensin receptor/neprilysin inhibitors (ARNi). These medications contain an angiotensin-receptor blocker (ARB) component and a neprilysin inhibitor component. Valsartan is an ARB that is indicated for the treatment of hypertension. It blocks the action of angiotensin II, resulting in less vasoconstriction of blood vessels. Sacubitril is a neprilysin inhibitor that blocks the breakdown of vasoactive peptides, such as bradykinin. This counteracts vasoconstriction and cardiac remodeling.
Previous studies showed that using ARBs and neprilysin inhibitors together was more beneficial than utilizing either medication on its own in heart failure treatment. Studies also found safety concerns with an increased risk of angioedema when studying angiotensin-converting-enzyme (ACE) inhibitors in combination with neprilysin inhibitors. Therefore, they were not used together in this study or in practice.
When this trial was published in 2014, ACE inhibitors were an essential treatment option for patients with HFrEF, because they had been shown to reduce mortality. ARBs showed lack of evidence for mortality reduction and were only recommended for use in patients who could not tolerate ACE inhibitors.
How was the study conducted?
The PARADIGM-HF trial had three main phases. After phase one and two, patients who could not tolerate the medication were removed from the study. The intention-to-treat population was analyzed with a total of 8399 patients split into the two treatment groups.
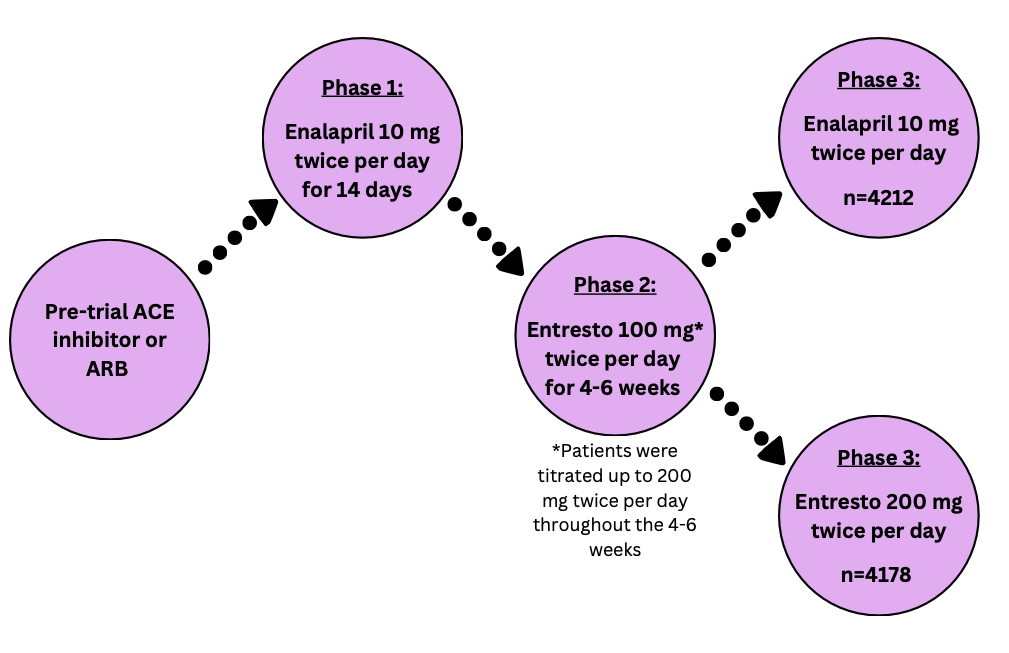
Patients enrolled in this study had moderate to severe disease and tolerated medication to treat heart failure. Patients excluded from this study had conditions that made it unsafe to take the treatment medications.
Notable* inclusion criteria:
- New York Heart Association Class II, III, or IV heart failure
- Ejection fraction of 35% or less
- Pre-study ACE inhibitor or ARB dose equivalent to 10 mg of enalapril or more
- Stable use of a beta blocker and ACE inhibitor or ARB for at least 4 weeks
Notable exclusion criteria:
- Hypotension, defined as a systolic blood pressure of <100 mmHg
- Estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73m2
- Previous angioedema or serious side effects to ACE inhibitors or ARBs
(*This list is not exhaustive and includes the highlights for patient enrollment parameters)
Many of the primary and secondary endpoints are reflective of common events that happen in patients with heart failure due to their decreased cardiac function.
The primary endpoint measured was a composite of:
- death from any cardiovascular cause
- heart failure symptoms causing hospitalization for the first time
Multiple secondary endpoints were measured including:
- Time from treatment to death from any cause
- Change in the Kansas City Cardiomyopathy Questionnaire (KCCQ) from baseline to 8 months
- New atrial fibrillation onset
- Renal function decline
During statistical analysis, the intention-to-treat population was analyzed using Cox’s proportional hazards model. Hazard ratios (HR) and confidence intervals (CI) were reported.
Outcomes of the trial
The PARADIGM-HF trial was stopped early due to overwhelming evidence that Entresto showed superior benefit.
The primary endpoint occurred in 21.8% of patients treated with Entresto and 26.5% of patients treated with enalapril. This outcome was statistically significant with a p-value <0.001, favoring the use of Entresto over enalapril.
All primary endpoint components were individually evaluated and found to be statistically significant in favor of Entresto. The secondary endpoints of death from any cause and changes from baseline in KCCQ scores were also statistically significant in favor of Entresto. The remaining secondary endpoints showed no significant differences between the two treatment groups.
The most common side effect reported for Entresto was hypotension. For enalapril, common side effects included cough, hyperkalemia, and decreases in eGFR. Overall, 10.7% of patients taking Entresto and 12.3% of patients taking enalapril stopped taking the medication due to side effects.
Entresto, a combination drug consisting of sacubitril and valsartan, was more effective than enalapril alone at reducing mortality from cardiovascular causes or hospitalizations due to heart failure for patients with HFrEF. The safety profiles of these two medications were similar with enalapril having a wider variety of reported side effects.
What does this mean for pharmacists?
The heart failure treatment guidelines have evolved over the last 10 years as data from the PARADIGM-HF trial and other landmark HF trials has been published. The table below shows the progression of the guidelines for treating patients with HFrEF.
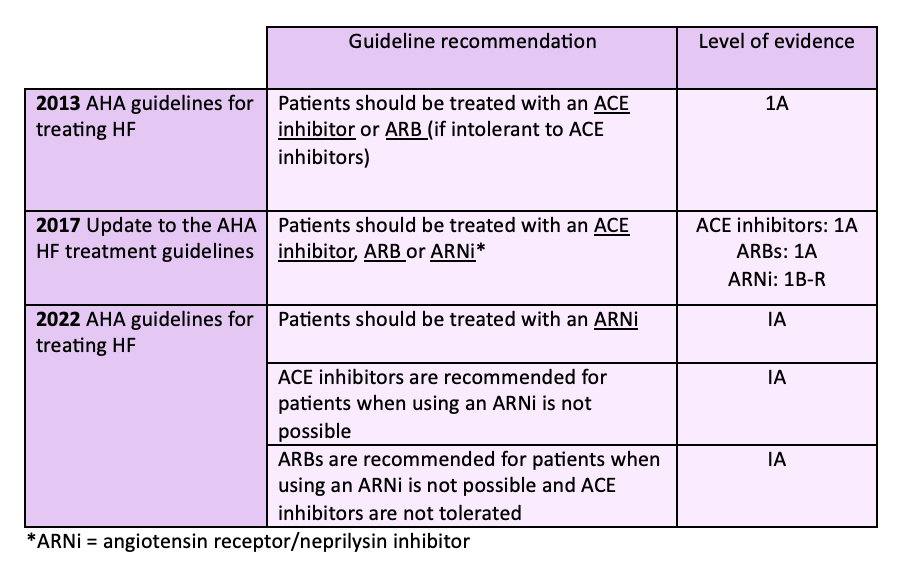
It is now recommended to use an ARNi for HFrEF treatment unless there are factors prohibiting its use. ACE inhibitors and ARBs are still safe and effective choices for treating patients especially those who may not be able to afford a brand name-only medication such as Entresto.
As pharmacists, it is important to stay up to date on landmark trials and the effect they have on treatment guidelines. The PARADIGM-HF trial showed less mortality and fewer hospitalizations for patients with HFrEF when using an ARNi compared to an ACE inhibitor. The outcome of this trial led to changes in the treatment guidelines for HFrEF to include a new class of medications that provide stronger benefits for patients.
Margaret M., APPE Student
References:
- Angiotensin-Neprilysin Inhibition versus Enalapril in Heart Failure. The New England Journal of Medicine. 2014;371:993-1004. DOI: 10.1056/NEJMoa1409077. Available at: https://www.nejm.org/doi/full/10.1056/NEJMoa1409077. Accessed August 21, 2024.
- 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Available at: https://pubmed.ncbi.nlm.nih.gov/23747642/. Accessed August 21, 2024.
- 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Available at: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000509. Accessed August 21, 2024.
- 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Available at: https://www.ahajournals.org/doi/full/10.1161/CIR.0000000000001063. Accessed August 21, 2024.
- Heart Failure Epidemiology and Outcomes Statistics. National Library of Medicine. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10864030/#:~:text=2.1.,develop%20HF%20in%20their%20lifetime. Accessed August 21, 2024.
