
Human immunodeficiency virus (HIV) is a retrovirus that can lead to acquired immunodeficiency syndrome (AIDs) if left untreated. Almost 40 million people worldwide have HIV and 630,000 people died from HIV in 2023. HIV attacks the immune system by targeting white blood cells. Patients infected with HIV are more likely to contract diseases such as tuberculosis and cancer due to their decreased immune function. Currently, there is no cure for HIV, but many medications exist to treat HIV and prevent its spread. The long-term effectiveness of these medications is directly related to patient adherence and treatment resistance. Long-acting injectable medications may be a good option for patients who struggle with adherence to daily oral treatments.
What was the FLAIR trial?
The FLAIR trial was a randomized, open-label, noninferiority trial taking place in multiple treatment centers. It aimed to prove noninferiority of an injectable two-drug antiviral regimen versus an established oral three-drug antiviral regimen in patients who had never received treatment for HIV.
The experimental medication, now known as Cabenuva, is a long-acting treatment containing two medications, both of which were individually FDA-approved for the treatment of HIV prior to this trial. The first is cabotegravir, an integrase strand-transfer inhibitor (INSTI). INSTIs work by blocking the integration of viral DNA, stopping the replication of HIV. The second medication is rilpivirine, which is a nonnucleoside reverse-transcriptase inhibitor (NNRTI). NNRTIs work by inhibiting HIV-1 transcriptase which prevents HIV replication. In this trial, Cabenuva was given as an intramuscular injection once monthly.
The comparator treatment was a standard HIV treatment regimen, containing three oral antivirals: dolutegravir, abacavir, and lamivudine. This combination is taken once daily and represents a guideline-recommended treatment option for HIV.
The goal of HIV treatment is to reduce morbidity and mortality as well as decrease the spread of HIV. Antiviral medications are used to reduce a patient’s viral load to an undetectable level, which prevents HIV transmission. As defined by the CDC, antiviral treatment that results in a viral load of < 200 copies per mL is considered to be successful.
How was the study conducted?
The FLAIR trial contained two main phases, the induction phase and the maintenance phase. In the induction phase, all enrolled patients were given the three-drug oral regimen daily for 20 weeks. After 16 weeks, patients underwent additional testing to ensure that their viral load was less than 50 copies per mL. Patients who met the criteria continued to the maintenance phase where they were randomized into either the experimental or comparator treatment group. Members in the experimental group were given an oral lead-in of daily cabotegravir and rilpivirine, followed by a loading dose injection, and then continued with the monthly injectable maintenance dose thereafter. Members in the comparator group continued on the daily three-drug oral regimen. The intention-to-treat population was analyzed with a total of 566 patients split evenly between the two treatment groups.
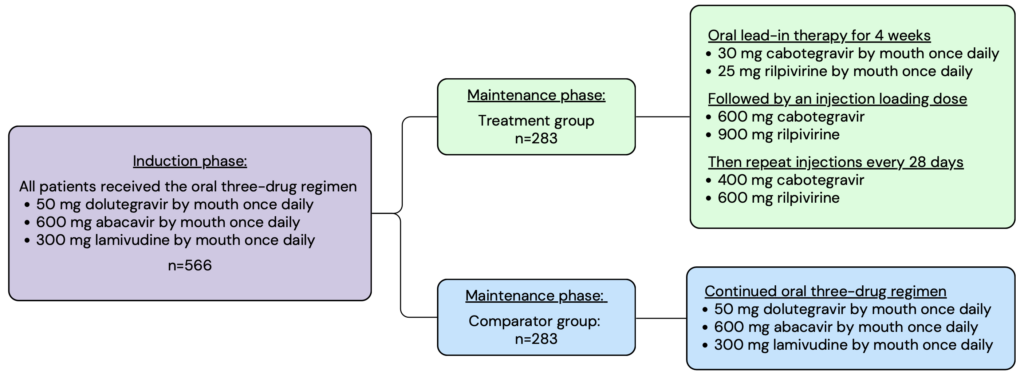
Patients included in the FLAIR trial had to have active HIV, as determined by a plasma screening, that had never been treated with antiviral medications. This is because resistance is common in patients who have previously been treated with antiviral medications. To avoid complicating the results, the trial only included patients who had no history of antiviral use and therefore had the lowest likelihood of treatment resistance. Patients were excluded from this trial if they could not take antiviral medications due to their comorbid health conditions.
Notable inclusion criteria:
- HIV infection that has never been treated with antiviral medications
- HIV RNA level ³ 1000 copies per mL
- 18 years old or older
Notable* exclusion criteria:
- Anyone who was pregnant or breastfeeding
- Patients with active Stage 3 disease as defined by the CDC
- Hepatic impairment
- Current or previous hepatitis B infection
*This list includes highlights from the patient enrollment parameters. It does not include all exclusion criteria.
The primary endpoint measured during this trial reflects the ability of the long-acting treatment and comparator medications to provide viral suppression, which is the main goal of HIV treatment. This was defined as the percentage of participants with a plasma HIV RNA level of ³ 50 copies/mL at week 48.
The secondary endpoints provide additional data regarding treatment effectiveness, adverse events, and pharmacokinetics. The key secondary endpoint measured was the percentage of patients with a plasma HIV RNA level of < 50 copies/mL at week 48.
Some additional secondary endpoints included:
- Virologic failure
- Adverse events
- Plasma pharmacokinetics
- Adherence
The intention-to-treat population was statistically analyzed. The adjusted difference between the treatment and comparator groups was determined for the primary and secondary endpoints using a stratified Cochran-Mantel-Haenszel analysis. Based on the power of the study and the size of each study group, noninferiority for the primary outcome was proven if the difference between the two groups was less than 6 percentage points.
Outcomes of the trial
The primary endpoint occurred for 6 patients in the Cabenuva group and 7 patients in the comparator group. This was a difference of -0.4 percentage points, demonstrating noninferiority of the study drug.
The key secondary endpoint occurred in 93.6% of patients in the Cabenuva group and 93.3% of patients in the comparator group, also demonstrating noninferiority of the treatment group with an adjusted difference of 0.4 percentage points (95% CI: -3.7 to 4.5).
Adverse events were more common in the Cabenuva group (94%) versus the comparator group (80%). The most common side effect of Cabenuva was injection site reaction, occurring in 86% of patients. Injection site pain accounted for most of these and was most commonly characterized as mild in severity. After the initial injection, 71% of patients reported injection-site reactions, and at week 48, the prevalence dropped to 20% of patients who received the trial medication. Additional common adverse effects included headache, diarrhea, and upper respiratory tract infection.
The FLAIR trial showed noninferiority of Cabenuva, a long-acting injectable medication when compared to a three-drug oral regimen to treat HIV. Cabenuva achieved similar rates of viral suppression for patients who had not previously been treated with antivirals for HIV. However, it also had higher rates of adverse effects. These were most commonly injection site reactions, which can be accounted for by the difference in the route of administration.
Another clinical trial, the ATLAS trial, studied the efficacy of Cabenuva in patients who had been successfully treated for HIV with antivirals in the past. Similar outcomes were reported for both the FLAIR and ATLAS trials. These two trials provided data supporting the efficacy of Cabenuva in viral suppression for patients with HIV regardless of their previous antiviral use.
Barriers to successful HIV treatment
HIV is an extremely complex disease state to treat. Many medications can be used in a variety of combinations to treat patients. The table below outlines a few common first-line treatment recommendations.
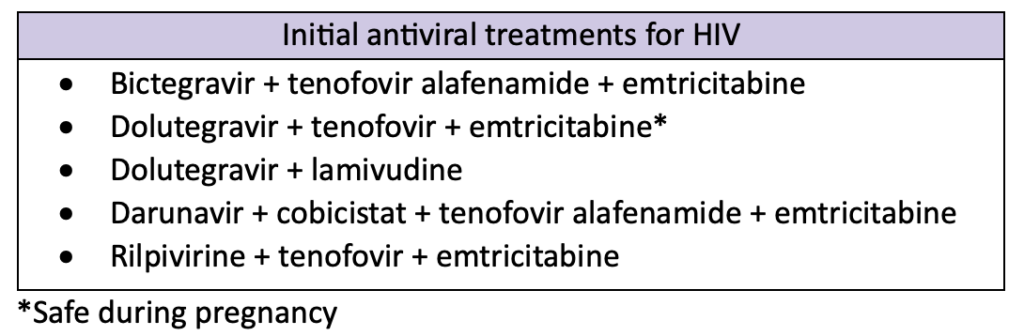
Regimens that contain bictegravir or dolutegravir are highly effective and tolerable. They also have a lower pill burden and high barrier to resistance compared to other medications, making them good options for initial HIV treatment. Treatment is extremely patient-specific and should take into consideration a variety of factors, including viral load, previous antiviral use, resistance, concurrent infections, and patient intolerance.
Cabenuva is now part of the HIV treatment guidelines. However, Cabenuva is not currently a first-line treatment option. It should not be used if the patient has any history of treatment failure or resistance to either cabotegravir or rilpivirine. Additionally, Cabenuva is not recommended for initial viral suppression. Patients should undergo successful viral suppression with an alternative treatment regimen before switching to a long-acting injectable medication.
Adherence to antiviral medications is key to HIV treatment. These medications reduce morbidity and mortality for patients as well as prevent the spread of HIV. However, their effectiveness is directly related to patient adherence. Poor adherence increases the risk of resistance to HIV medications, limiting treatment options. Barriers to patient adherence include pill burden, complex dosing, side effects, limited access to care, stigma, poor health literacy, and more. Long-acting injectable medications could improve adherence by eliminating pill burden and reducing complex dosing schedules. However, they come with unique side effects and require monthly appointments for administration.
Treatment resistance is a major problem that occurs with HIV medications. Patients should undergo genotype testing for antiviral resistance before starting treatment. This step is of greater importance for patients who acquire HIV while using pre-exposure prophylaxis (PrEP), because some of the medications for PrEP and HIV treatment overlap. Poor adherence is a common cause of treatment resistance. However, resistance can develop even with perfect adherence. Virologic failure (HIV RNA plasma level > 200 copies/mL) due to treatment resistance often requires patients to switch treatment regimens. As patients acquire resistance to first-line treatments, they may have to switch to more complex regimens.
Pharmacists play an important role in HIV care by educating patients on treatment options and encouraging adherence.
Margaret M., APPE Student
References
- Long-Acting Cabotegravir and Rilpivirine after Oral Induction for HIV-1 Infection. The New England Journal of Medicine vol. 382,12 (2020): 1124-1135. doi:10.1056/NEJMoa1909512. Available at: https://pubmed.ncbi.nlm.nih.gov/32130806/. Accessed August 29, 2024.
- About HIV. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/hiv/about/index.html. Accessed August 29, 2024.
- HIV and AIDS. World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids#:~:text=Human%20immunodeficiency%20virus%20(HIV)%20is,cells%2C%20weakening%20the%20immune%20system. Accessed August 29, 2024.
- The Importance of Treatment Adherence in HIV. AJMC. Available at: https://www.ajmc.com/view/a472_sep13_schaecher_s231. Accessed August 29, 2024.
- Antiretroviral Drugs for Treatment and Prevention of HIV Infections in Adults: 2022 Recommendations of the International Antiviral Society – USA Panel. International Antiviral Society. Available at: https://pubmed.ncbi.nlm.nih.gov/36454551/. Accessed August 30, 2024.
