Pharmaceutical compounding is an important part of the care that pharmacy staff provide for patients daily. The United States Pharmacopeia (USP) sets national standards for nonsterile and sterile pharmacy compounding in USP <795> and USP <797> respectively. These standards outline the information needed to compound a safe pharmaceutical product, from training requirements to personal garbing, to beyond-use dates, and more. On November 1, 2022, USP issued major updates to USP <795> and USP <797>. Pharmacies around the country had until November 1, 2023, to put these changes into practice. As a pharmacist, it is important to understand and implement the most updated USP guidelines to ensure the accuracy and safety of all the products the pharmacy generates. Reviewing these major changes will also be critical for pharmacists studying for licensure!
Why where USP <795> and <797> changed?
USP <795> and <797> were updated for the following reasons:
- To reflect improvement in pharmacy practice and science
- To provide clarification on information that was commonly misunderstood
- To integrate ideas from stakeholders
Updates to USP <795> for nonsterile pharmacy compounding
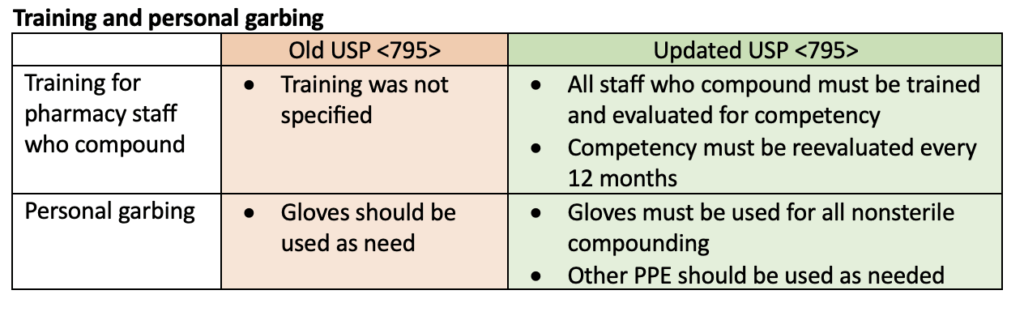
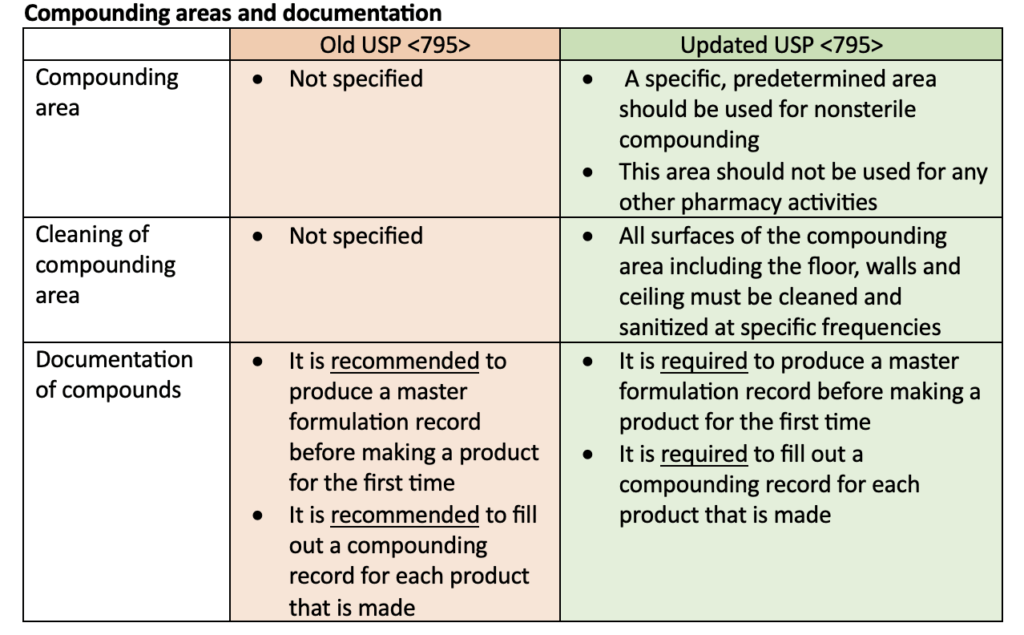
Multiple updates were made to USP <795> including training and personal garbing requirements, specifications for compounding areas, instructions for using master formulation and compounding records, and changes to beyond-use dates (BUDs). The following tables highlight some of the updates to the USP <795> requirements.
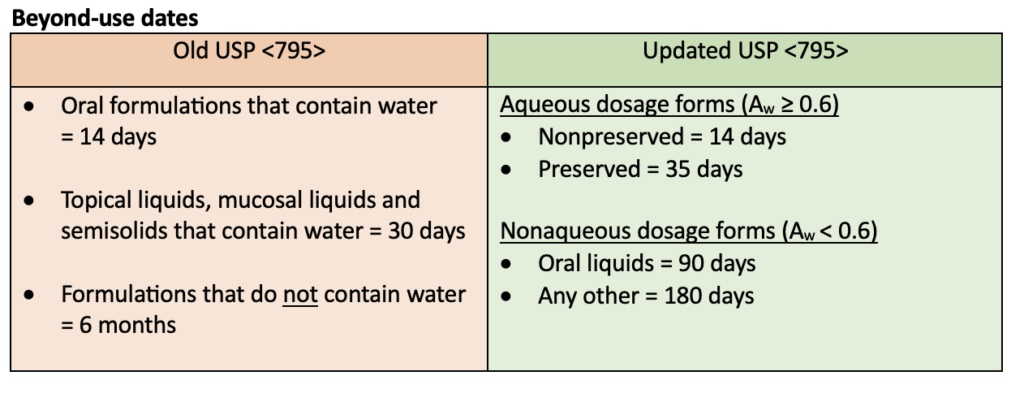
BUDs were the biggest change when USP <795> was updated in 2022. Nonsterile compounds are now defined by their water activity instead of being categorized as nonaqueous or water-containing. Water activity (Aw) is used to determine how likely contamination by microbes and/or degradation by hydrolysis is to occur for nonsterile compounded products. Aqueous products have an Aw ≥ 0.6 and nonaqueous products have an Aw < 0.6.
Updates to USP <797> for sterile pharmacy compounding
Many updates were made to USP <797>. The most notable update was a change in the definition of microbial contamination categories for sterile compounded products. This definition change was reflected in many of the other updates including personal garbing, aseptic technique evaluations, and beyond-use dates. Requirements for master formulation records and compounding recorders were also specified. The following tables compare the old USP <797> requirements to the new USP <797> requirements but do not represent all the changes made.
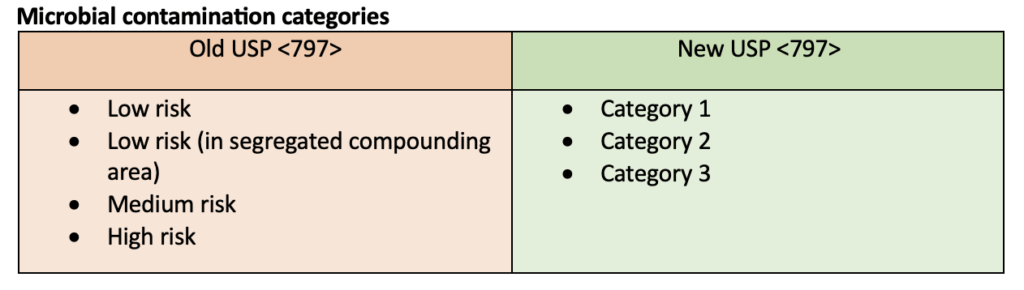
- Category 1 compounds must be prepared in at least an ISO Class 5 area that is placed in a segregated compounding area. These compounds require the least amount of environmental control.
- Category 2 compounds must be prepared in a cleanroom and require more control of the environment than category 1 compounds.
- Category 3 compounds must be prepared in a cleanroom. They undergo additional sterility testing and have added requirements for the area in which they are compounded so that they can have longer BUDs. Those requirements include additional training, sterile garb, cleaning, and monitoring of the environment.
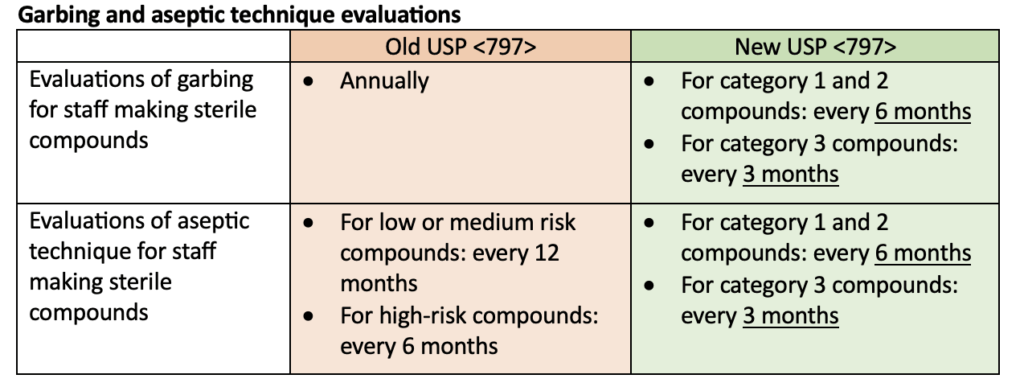
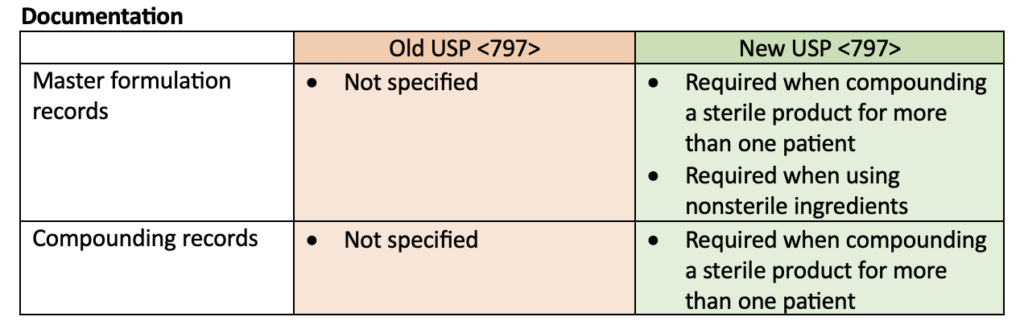

Putting these changes into practice!
USP <795> and <797> have recently been updated to improve standards of practice in compounding. These changes encompass a wide variety of updates from personal garbing to beyond-use dates. It is important to implement and follow these new guidelines in every pharmacy that provides compounding services for the health and safety of all patients.
Margaret M., APPE Student
References
- USP Compounding Standards and Beyond-Use Dates. United States Pharmacopeia. Available at: https://www.usp.org/sites/default/files/usp/document/our-work/compounding/usp-bud-factsheet.pdf. Accessed August 20, 2024.
- USP <795> Key Changes. American Society of Health-System Pharmacists. Available at: https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/compounding/docs/USP-795-Key-Changes.pdf. Accessed August 20, 2024.
- USP <797> Key Changes. American Society of Health-System Pharmacists. Available at: https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/compounding/docs/USP-797-Key-Changes.pdf. Accessed August 20, 2024.
- <795> FAQs. United States Pharmacopeia. Available at: https://go.usp.org/USP_GC_795_FAQs?_gl=1*3djyew*_gcl_au*MTEyNjE5NjUxMi4xNzI0MTYwODgz*_ga*MTEzMjYzODUzOC4xNzI0MTYwODgz*_ga_DTGQ04CR27*MTcyNDE2MDg4My4xLjEuMTcyNDE2NjU3Mi4wLjAuMA. Accessed August 20, 2024.
- <797> FAQs. United States Pharmacopeia. Available at: https://go.usp.org/USP_GC_797_FAQs?_gl=1*xwon9t*_gcl_au*MTEyNjE5NjUxMi4xNzI0MTYwODgz*_ga*MTEzMjYzODUzOC4xNzI0MTYwODgz*_ga_DTGQ04CR27*MTcyNDE3MjQ4NS4yLjAuMTcyNDE3MjQ5MC4wLjAuMA. Accessed August 20, 2024.
- Revisions to USP Compounding Standards <795> and <797>. National Community Pharmacist Association. Available at: https://ncpa.org/sites/default/files/2022-11/2022-ncpa-usp-presentation.pdf. Accessed August 20, 2024.
