
The FDA recently approved the first over-the-counter (OTC) birth control pill, Opill. Opill’s active pharmaceutical ingredient was previously approved as a prescription-only medication. Still, given the absence of prescriber oversight, pharmacists must be especially prepared to counsel and answer questions about this medication.
What is the new OTC birth control?
Opill is a daily progestin-only pill (POP) that can be used to prevent pregnancy. It is the first and only oral birth control pill to be available over the counter. Opill is very similar to other POPs in both ingredients and mechanism of action. The main difference between Opill and other POPs is that Opill does not require an appointment with a provider or a prescription. It can be purchased at many drugstores and online.
About Opill
The active pharmaceutical ingredient in Opill is 0.075mg of norgestrel.
Opill’s mechanism of action is the same as other POPs. It works by:
- Causing the cervical mucus to thicken
- Slowing ovulation
- Lowering the LH and FSH peaks
The most common side effects of Opill and other POPs are:
- Irregular, breakthrough bleeding between periods
- Headaches
- Weight gain and/or increased appetite
- Nausea
- Abdominal cramping
Cost: A 28-day supply of Opill costs $19.99.
When to use POPs such as Opill
A benefit of POPs is that they do not contain estrogen. This makes them a favorable option for patients who want to take an oral contraceptive but have a contraindication to estrogen-containing products such as combined hormone contraceptives (CHCs).
Contraindications to CHCs include:
- Hypertension that is not controlled
- Smoking more than 15 cigarettes per day (if the patient is over 35 years old)
- Migraines with aura
- History of stroke
- History of venous thromboembolism
- Breast cancer or history of breast cancer
- Endometrial cancer
- Valvular heart disease
- ASCVD
- Liver cirrhosis
- Diabetes with microvascular complications
One of the main risks of CHCs is venous thromboembolism (VTE) due to the estrogen component of the medication. Estrogen increases blood coagulability which increases the patient’s risk of VTE. Since POPs do not contain estrogen, they do not present the same clotting risk and are safer for OTC use.
When not to use POPs such as Opill
It is also important to understand when POPs are contraindicated and should not be used.
POP contraindications include:
- Uterine bleeding that is atypical and undiagnosed
- Breast cancer or a history of breast cancer
- Previous bariatric surgeries
- Liver cirrhosis
- Coadministration with certain medications to treat seizures, HIV, or tuberculosis
Important counseling points
Most norethindrone-based POPs, such as Opill, do not have any placebo tablets. Therefore, it is critical that patients do not skip any of the tablets.
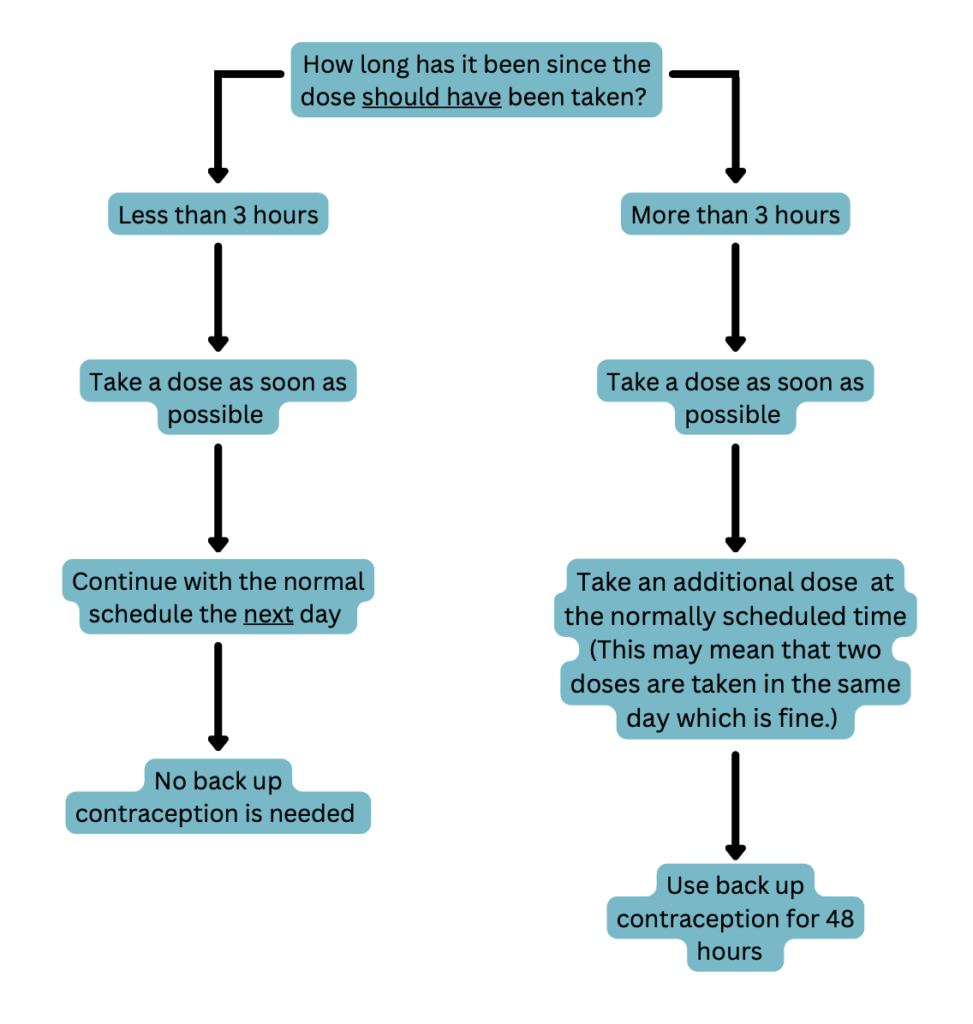
POPs are very time-sensitive due to their short half-lives of about 8 hours. Thus, it is important to remind patients to take their tablet at the same time each day to keep their contraceptive concentrations consistent. Encourage them to set a reminder on their phone or take this medication at the same time as another daily activity such as eating breakfast or dinner. If an individual misses a dose they should proceed in 1 of 2 ways depending on how many hours they are past the time they normally take the tablet. See the following flowchart for details.
Patients should see a medical provider if they develop pain in their lower abdomen, migraines with aura, or jaundice while taking POPs. They should also see a medical provider if they become pregnant or develop intolerable side effects.
An additional form of contraception, such as condoms, should be used for 48 hours after starting POPs and any time a patient is 3 or more hours late taking a dose. Other forms of hormonal birth control should not be used as backup contraception for POPs.
Birth control pills, including POPs and CHCs, are about 93% effective making them more effective than barrier methods (87% effective) but less effective than implants such as IUDs (99% effective). Like most birth control, the effectiveness of POPs is directly dependent on the individual’s ability to take the medication as directed. This means taking the medication at the same time each day and never missing a dose.
Because the effectiveness of birth control pills is directly related to medication adherence, pharmacists need to discuss all birth control options with patients to help them decide if an OTC option is best or if they should see a provider for a prescription-only option. OTC birth control pills may not be the best choice for everyone. For example, someone who is unable to adhere to a strict dosing schedule may require a prescription-only alternative, such as an implant. On the other hand, OTC birth control pills may be a great option for people who have limited access to healthcare providers and may not be able to get a prescription.
What does this mean for pharmacists?
Opill, the new over-the-counter birth control pill, was recently FDA-approved and is now being sold in many drug stores. This medication is very similar to prescription POPs with the biggest difference being its OTC status. Neither an appointment with a prescriber, nor a prescription is necessary to start taking Opill. This provides an accessible option for patients who have limited access to healthcare providers. Although increased accessibility is important, birth control pills do come with risks and contraindications. Pharmacists need to be ready to have risk versus benefit discussions about all birth control options and help patients choose the option that is best for them. Pharmacists should also be prepared to counsel and answer questions about Opill, because they are likely to be the main healthcare providers advising patients who are considering this medication.
Margaret M., APPE Student
References
- Oral Contraceptive Pills. National Library of Medicine. Available at: https://www.ncbi.nlm.nih.gov/books/NBK430882/. Accessed August 19, 2024.
- Progestin-Only Hormonal Birth Control: Pill and Injection. American College of Obstetricians and Gynecologists. Available at: https://www.acog.org/womens-health/faqs/progestin-only-hormonal-birth-control-pill-and-injection. Accessed August 19, 2024.
- Opill tablet. Package Insert. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017031s035s036lbl.pdf. Accessed August 19, 2024.
- Birth Control. Planned Parenthood. Available at: https://www.plannedparenthood.org/learn/birth-control. Accessed August 19, 2024.
- Opill. Opill Promotional Website. Available at: https://opill.com/products/opill?variant=48920420286768. Accessed August 19, 2024.
