Empowering Health: The Role of Pharmacy in Health Screenings
There’s a certain Latin saying that goes, “It is better and more useful to meet a problem in time than to seek a remedy after the damage is done.” If you’re not familiar with it, don’t fret. You’re probably more familiar with the short and sweet version of it: “Prevention is better than cure”, said by a certain Dutch philosopher. Well, however old the saying may be, it’s certainly timeless.
Prevention is the cornerstone of good health, and health screenings play a big part in that by identifying potential health concerns early on. Pharmacists play a vital role in health screenings that may commonly be observed in communities. We’ll dive into the importance of health screenings, the different types of screenings, and the role that pharmacists play in these scenarios.
Why Health Screenings Matter
As commonly said in healthcare, “the earlier the detection, the better the health outcome.”1 Partaking in health screenings is a proactive step towards taking care of oneself. The primary advantage lies in the ability to catch potential health concerns before they become problematic. By identifying risk factors and early signs of conditions, individuals take matters into their own hands by making such decisions and taking action to improve their health.
Types of Health Screenings
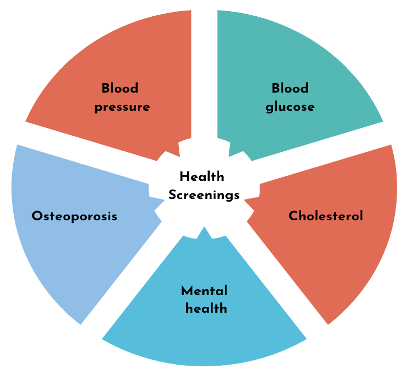
There are various health screenings available to patients that are offered by pharmacies. The most common screenings include blood glucose testing, blood pressure monitoring, and total cholesterol checks. Other health screenings that are not usually offered at pharmacies but still utilize involvement from pharmacists are for mental health conditions, osteoporosis, opioid use disorders, and sexually transmitted disease (STDs).2,3
Ask The Pharmacist for Help
Pharmacists are well equipped to guide patients through the entire health screening process. They are allies when it comes to disease prevention, especially for the most common health conditions of diabetes and blood pressure. Not only are pharmacists able to help patients understand what their results mean, but they are able to recommend certain lifestyle changes and even provide counsel on necessary follow-up steps for further care. Pharmacists are trusted healthcare professionals and easily accessible to patients when it comes to who to ask regarding their medications AND when it comes to their health as a result of health screenings.3
The Bottom Line
Through early detection, education, and collaboration with patients and healthcare professionals, pharmacists can help manage and prevent health issues for all patients. Patients themselves can take matters into their own hands and improve their own health by taking the necessary steps for management such as partaking in health screenings. Moreover, pharmacies can continue to be known not only as the place to get medications from, but also the place to seek out for taking steps toward disease prevention. Being proactive will pay off in the long run, so don’t hesitate to get that screening and talk to the pharmacist. Your future self may thank you one day.
Midrara Kashmari
RxPharmacist Team
References:
- Givler DN, Givler A. Health Screening. Nih.gov. Published 2019. https://www.ncbi.nlm.nih.gov/books/NBK436014/.
- Get Screened – MyHealthfinder | health.gov. health.gov. https://health.gov/myhealthfinder/doctor-visits/screening-tests/get-screened.
- Jennifer Gershman P. Pharmacists Play Vital Role in Health Screenings. wwwpharmacytimescom. 2022;88. https://www.pharmacytimes.com/view/pharmacists-play-vital-role-in-health-screenings.
Empowering Health: The Role of Pharmacy in Health Screenings Read More »